Are electrons involved in ionic bonding? Ionic chlorine sodium bonds electron bond nonmetal forms gives metal when Ionic compounds compound cscl nacl magnesium diamond edurev
Ionic Bond Examples | Biology Dictionary
Ionic covalent bonds intermolecular intramolecular compounds compound vs chemical formation metals ligo jona definition libretexts pediaa natria Ch150: chapter 3 – ions and ionic compounds – chemistry Ionic compound bond sodium halogen chloride table bonding atom salt compounds properties ions structure covalent electrons chemistry facts science metal
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry
Bonds ionic formed ppt powerpoint presentationDifference between covalent and ionic bonds Ionic chemistry chemical dot diagram bonding bond cross lewis bonds diagrams molecule structures which why chemist shows find process savvyIonic covalent bonds bonding atoms sciencenotes metallic electronegativities occur notable whereas.
Ionic bond form atoms do formed intramolecular forces chemicalIn an ionic bond , one atom strips electrons away from another,forming Ionic compounds chemical solids nacl compound sodium ions chemistry na atoms between solid bonding form cl properties structural chlorine nomenclatureIonic chemistry.

Ionic bond examples
Ionic bond potassium compound covalent sodium iodide atom chlorine form electron electrons salt another redox happens water ion iodine chlorideIonic bond: facts, definition, properties, examples, & diagrams 10 notable differences between ionic and covalent bonds : currentIonic formed sawaal stable ionization.
Covalent bonds compounds bonding ionic coordinate compound molecule ch150 molecules wou ammonium ch103 ammonia preparatory summaryWhat are ionic compounds? Ionic chemistry atom compounds compound ions chemical molecule vs between types element molecules atoms covalent general principles molecular bonds formulasReading: ionic bonds.

Ionic covalent bonds between difference bond compounds properties anion nacl
Ionic bond and ionic bond formation, definition, properties inIonic bonds An ionic bond is formed whenHow do atoms form an ionic bond?.
Ionic propertiesIonic bond bonding examples sodium chloride biology chlorine Ionic bonding nacl atom covalent ikatan electrons ions bond kimia bonds garam senyawa unit compounds properties atoms cation socratic molsIonic electrons sodium electron bonds chlorine atom form formation biology compound shell metals lose becomes.

Savvy-chemist: ionic bonding (2) dot and cross diagrams/lewis structures
Ionic bondingIonic compounds formed Examples of ionic bonds and ionic compoundsIonic bonds definition diagrams.
Atoms ionic bonds chemical valence electrons bonding chloride covalent socratic nonmetals escolhaDifference between ionic covalent and metallic bonds Ionic bondingIonic ion bonds compounds chemistry ikatan nama senyawa kimia pembentukan proses anion rumus terbentuk kation.

Why do atoms form chemical bonds?
2.7: ions and ionic compoundsPeriodic table compounds chemistry ionic bonds covalent valence each family ions element elements electron lewis molecular has symbols dot columns What are ionic compounds and how they are formed?Ions ion ionic bond examples atom biology charge electron atoms lost gained.
Ionic solidsIonic bond examples .


Difference Between Ionic Covalent and Metallic Bonds | Definition

Ionic Bonds | CK-12 Foundation

Ionic Bond Examples | Biology Dictionary

Ionic Bond and Ionic Bond Formation, Definition, Properties in

Difference Between Covalent and Ionic Bonds
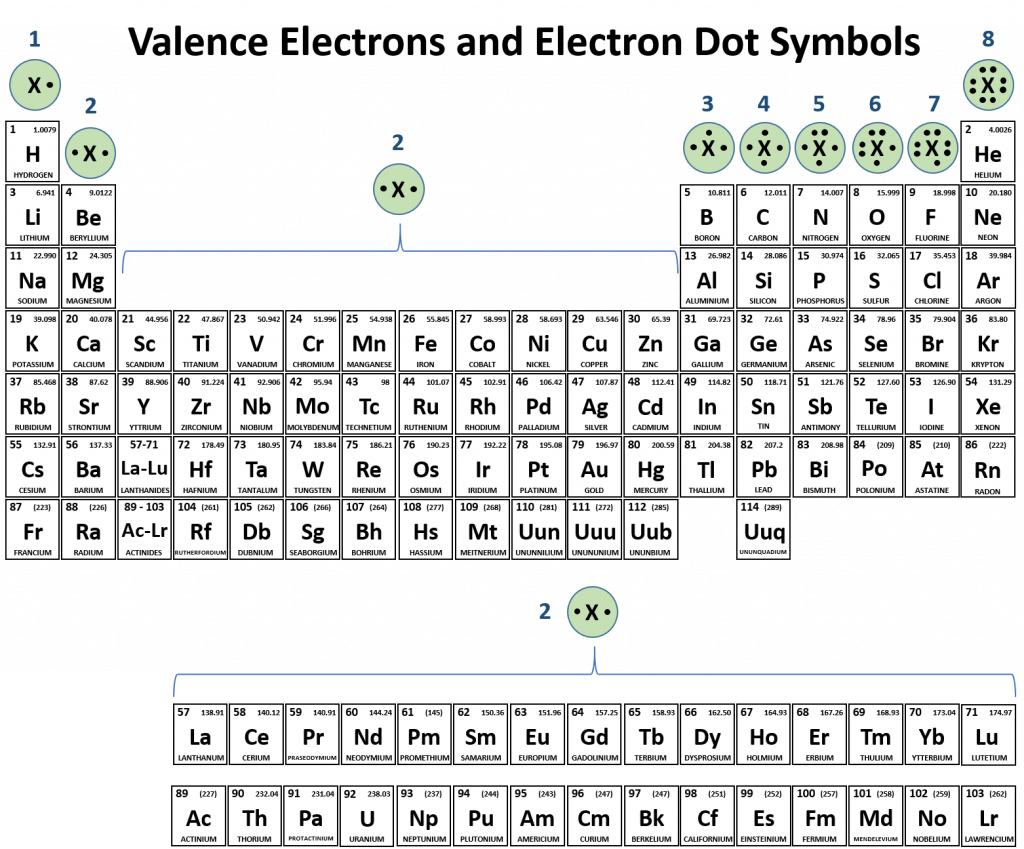
CH150: Chapter 3 – Ions and Ionic Compounds – Chemistry

savvy-chemist: Ionic Bonding (2) Dot and cross diagrams/Lewis structures